Step-by-step explanation:
The given reaction is as follows.
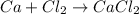
The number of elements on reactant side are as follows.
The number of elements on product side are as follows.
Therefore, the equation is completely balanced.
Thus, we can conclude that only one formula units of calcium chloride must be part of the products.