The reaction is of order three with respect to the reactant.
Step-by-step explanation
The rate of a reaction of order n about a certain reactant is proportion to the concentration of that reactant raised to the n-th power. This is true only if concentrations of any other reactants stay constant in the whole process.
In other words, Rate = constant × [Reactant]ⁿ, Rate ∝ [Reactant]ⁿ. (The symbol "∝" reads "proportional to".)
In this question,
[4 × Reactant]ⁿ ÷ [Reactant]ⁿ = 64.
In other words, 4ⁿ = 64, where n is the order of the reaction with respect to this reactant.
It might take some guesswork to find the value of n. Alternatively, n can be solved directly with a calculator using logarithms. Taking natural log of both sides:
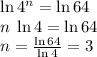 .
.
Evaluating
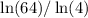 on Google or on a calculator with support for ln (the natural log) will give the value of n- no guesswork required.
on Google or on a calculator with support for ln (the natural log) will give the value of n- no guesswork required.
n = 3. Therefore, the reaction is of order three with respect to this reactant.