Answer:
642.6 kJ
Step-by-step explanation:
Using the formula for heat energy required:
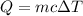 ,
,
where:
C → specific heat capacity = 1.68 kJ/kg°C
Q → heat energy required = ?
m → mass of the object = 4.5 kg
ΔT → change in temperature = 97°C - 12°C = 85°C,
Q = 4.5 x 1.68 x 85
= 642.6 kJ