Answer:
C.
Step-by-step explanation:
Specific heat capacity is defined as the amount of heat energy needed to increase the temperature of 1kg of a substance by 1°C.
The formula for specific heat capacity is as follows:
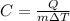
where:
C → specific heat capacity
Q → heat energy required
m → mass of the object
ΔT → change in temperature.