Answer : The amount of lithium nitrate will be, 438.755 g
Solution : Given,
Mass of lithium sulfate = 350 g
Molar mass of lithium sulfate = 109.94 g/mole
Molar mass of lithium nitrate = 68.9 g/mole
First we have to calculate the moles of lithium sulfate.
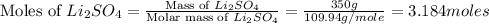
Now we have to calculate the moles of lithium nitrate.
The given balanced reaction is,
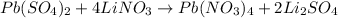
From the reaction we conclude that
2 moles of lithium sulfate produces from 4 moles of lithium nitrate
3.184 moles of lithium sulfate produces from
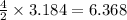 moles of lithium nitrate
moles of lithium nitrate
Now we have to calculate the mas of lithium nitrate.
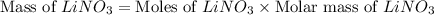
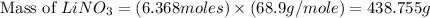
Therefore, the amount of lithium nitrate will be, 438.755 g