Answer: The Enthalpy of combustion of 1 mol of butane is -2657.5 kJ/mol.
Step-by-step explanation:
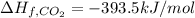
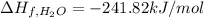

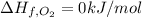
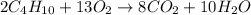
Enthalpy of Combustion of 2 moles of butane :
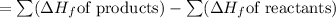
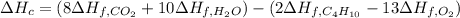
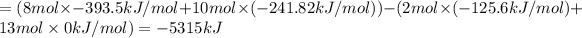
Enthalpy of Combustion of 1 moles of butane :
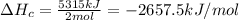
The Enthalpy of combustion of 1 mol of butane is -2657.5 kJ/mol.