Answer: Option (B) is the correct answer.
Step-by-step explanation:
A neutralization reaction is defined as a reaction in which an acid chemically reacts with a base in order to produce salt and water.
For example,
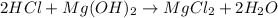
In this reaction, HCl is the acid and
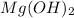 is the base. The salt produced by chemical combination of HCl and
is the base. The salt produced by chemical combination of HCl and
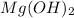 magnesium chloride.
magnesium chloride.
Therefore, we can conclude that products of the neutralization reaction between hydrochloric acid (upper H upper C l) and magnesium hydroxide are
 and
and
 .
.