Answer:
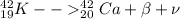
Step-by-step explanation:
In Beta decay unstable nuclei will convert into stable nuclei by converting its neutron into an electron and a proton.
this process is given as
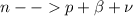
so in this process mass number will remain same but atomic number will increased by 1
So the new nuclei is of higher atomic number
So we will have
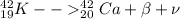
so above is the nuclear reaction