Answer: d. it is double
Explanation:
Boyle's Law: This law states that pressure is inversely proportional to the volume of the gas at constant temperature and number of moles.
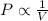 (At constant temperature and number of moles)
(At constant temperature and number of moles)
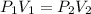
where,
 = initial pressure of gas = p atm
= initial pressure of gas = p atm
 = final pressure of gas = ?
= final pressure of gas = ?
 = initial volume of gas = v
= initial volume of gas = v
 = final volume of gas =
= final volume of gas =
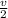
Now put all the given values in the above equation, we get the final pressure of gas.
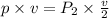
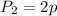
Therefore, the pressure of gas will be twice the original pressure.
The partial pressure of the gas depends on Total pressure and thus the partial pressure will also double.