Answer: The correct answer to this is it is a double replacement reaction because calcium chloride and sodium carbonate exchange their non-metallic parts.
Step-by-step explanation:
- Single displacement reaction is a type of chemical reaction in which more reactive metal displaces the less reactive metal from a chemical reaction. equation follows:

A is more reactive metal than B.
- Double displacement reaction is a type of chemical reaction in which exchange of ions takes place. Equation follows:
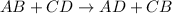
Reaction between calcium chloride and sodium carbonate follows:
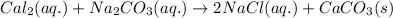
Here, a precipitate of calcium carbonate is formed because in this reaction exchange of anions took place. Chloride ion forms more stable compound with sodium rather than calcium metal. So, this reaction lead to the formation of sodium chloride and calcium carbonate.
Hence, this is a type of double displacement reaction because of the exchange of non-metallic parts in a given reaction.