Answer: 462.00 Joules of heat will be needed to change the temperature of copper from 20° C to 60° C.
Step-by-step explanation:
Mass of copper,m = 30.0 grams
Specific heat capacity of copper,c =
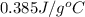
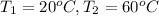
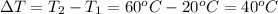
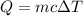
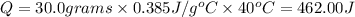
462.00 Joules of heat will be needed to change the temperature of copper from 20° C to 60° C.