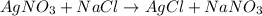Answer : The correct option is,
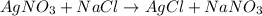
Explanation :
Precipitation reaction : It is defied as the chemical reaction in which the two soluble salts solutions combined to form an insoluble salts.
(1)
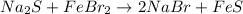
(2)
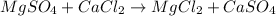
(3)
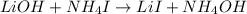
(4)
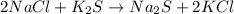
(5)
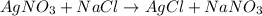
In the given reactions 1, 2, 3 and 4, the salt forms are completely soluble in water.
As we know that all the sodium, potassium and ammonium salts are soluble in water.
While in the reaction 5, the salt form that is silver chloride (AgCl) are insoluble in water. So, this reaction is a precipitation reaction.
Hence, the correct option is,