Answer : The correct option is, (d) 31
Explanation : The given atom is,
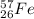
Given :
Atomic mass number = 57
Atomic number = 26
Atomic number = Number of protons = Number of electrons = 26
Now we have to calculate the number of neutrons.
Number of neutrons = Atomic mass number - Atomic number
Number of neutrons = Atomic mass number - Atomic number = 57 - 26 = 31
Hence, The total number of neutrons present in an atom of
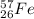 is, 31 neutrons.
is, 31 neutrons.