Answer : The correct option is, Neutrons.
Explanation :
The given atoms are,
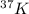 and
and
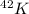
As from the periodic table, the atomic number of potassium is, 19
In
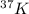 ,
,
Atomic mass number = 37
Atomic number = 19
Atomic number = Number of electrons = Number of protons = 19
Number of neutrons = Atomic mass number - Atomic number = 37 - 19 = 18
In
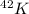 ,
,
Atomic mass number = 42
Atomic number = 19
Atomic number = Number of electrons = Number of protons = 19
Number of neutrons = Atomic mass number - Atomic number = 42 - 19 = 23
From this we conclude that the number of protons and electrons are same in given atoms but the number of neutrons are different.
Hence, an atom of potassium-37 and an atom of potassium-42 differ in the total number of Neutrons.