Answer : A metal in group 16 of the periodic table can become a chemically stable ion by gaining 2 electrons.
Explanation :
The group 16 element are oxygen, sulfur, selenium, tellurium and polonium.
The general electronic configuration of group 16 elements is,
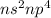
As we now that there are total 6 electrons in p sub-shell. So, a metal in group 16 can become a chemically stable ion by gaining 2 electrons.

Hence, a metal in group 16 of the periodic table can become a chemically stable ion by gaining 2 electrons.