The limiting reagent : HCl
Further explanation
Given
Mg + 2HCI - MgCl₂ + H₂
2.5 mol of Mg and 4.0 mol of HCl
Required
the limiting reagent
Solution
A method that can be used to find limiting reactants is to divide the number of moles of known substances by their respective coefficients
mol ratio Mg : HCl =
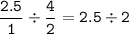
HCl as a limiting reagent(smaller ratio)