Answer:
a. 3.79e5 J
b. Decrease.
Step-by-step explanation:
Hello!
a. In this case, since the heat involved during a compression-expansion isothermal process is computed via:
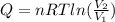
Now, since the final volume is one third of the initial one:
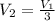
So we can plug in now:
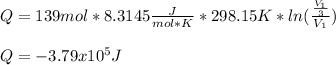
b. In this case, the relationship between initial and final volume is:
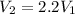
So the heat interaction is now:
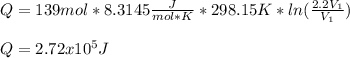
It means that the heat interaction decrease on the contrary process, it means that in a. heat was released by 3.79e5 J and in b heat is absorbed by 2.72e5 J.
Best regards!