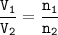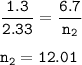The number of moles : 12.01
Further explanation
Given
V₁ = 1.3 L contains 6.7 mol of gas(n₁)
V₂ = 2.33 L
Required
The number of moles
Solution
Avogadro's : at the same temperature and pressure, the ratio of gas volume will be equal to the ratio of gas moles