Mass of Hydrogen atoms : 18 g
Further explanation
Given
73 gram of HCl
144gram of H₂O
Required
Mass (gram) atom of Hydrogen
Solution
H atoms are in 2 compounds, H₂O and HCl, so we calculate their mass in each compound
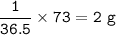
mass H in HCl :
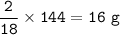
total mass = 2 g+ 16 g = 18 g