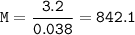Assuming gas temperature = 25, 3.2 g of a gas at 25°C occupy the same volume as 0.6266 g of CH4 at 17°C at constant pressure
The molecular mass of the gas : 842.1 g/mol
Find the molecular mass of the gas.
Further explanation
Given
3.2 g of gas
0.6266 g of CH4
Required
the molecular mass of the gas
Solution
Because V, P, and R are the same, from ideal gas law :
PV=nRT
n₁T₁=n₂T₂
n₂=moles CH₄= 0.6266 : 16 g/mol = 0.0392
T₁=25+273=298 K
T₂=17+273=290 K
mol of gas :
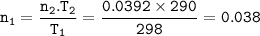
molecular mass :