Step-by-step explanation:
We need to write the work equation of the following chemical equation "hydrogen + oxgyen =water"
Hyrodgen is denoted by H₂ and Oxygen is denoted by O₂. The reaction between hydrogen and water is :
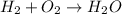
Now, we can balance this equation as follows :
On LHS,
Hydrogen = 2 atoms
Oxygen = 2 atoms
On RHS,
Hydrogen = 2 atoms
Oxygen = 1 atoms
To make it balance, multiply 2 to H₂ at LHS and 2 to H₂O at RHS.
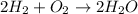
Now, no of atoms of hydrogen and oxygen at LHS are 4 and 2 respectively while at RHS no of atoms of hydrogen and oxygen are also 4 and 2 respectively.