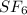Answer:
Step-by-step explanation:
Let the compound be x.
Given the following data;
Sulfur, S = 3.21g
Fluorine, F = 11.4g
Atomic mass of sulfur = 32.07g
Atomic mass of fluorine = 19g
Amount of moles for sulfur;
3.21*(1/32.07) = 0.10mol
Amount of moles for fluorine;
11.4*(1/19) = 0.60mol
We then divide by the smallest to find the ratio;
(0.10/0.10) = 1 Mol of sulfur.
(0.60/0.10) = 6 Mol of fluorine.
Therefore, the ratio of sulfur to fluorine is 1:6.
Compound x =
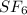
Hence, the empirical (simplest) formula of the compound is