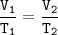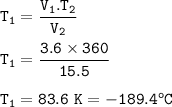Initial temperature = -189.4 °C
Further explanation
Given
the volume of a gas is changed from 3.6 L to 15.5 L
final temperature = 87 °C = 87+ 273 = 360 K
Required
initial temperature
Solution
Charles's Law
When the gas pressure is kept constant, the gas volume is proportional to the temperature