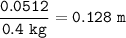The molality of a solution : 0.128 m
Further explanation
Given
15.9 g of Ca3(PO4)2 in 400 grams of water.
Required
The molality
Solution
molality = mol solute / kg solvent
solute= Ca3(PO4)2(MW=Molecular Weight: 310.2 g/mol)
mol solute :
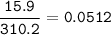
The molality :