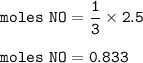Moles of NO formed : 0.833
Further explanation
Given
2.5 moles of NO2
Required
moles of NO formed
Solution
Reaction
3 NO₂(g) + H₂O(l) = 2 HNO₃(aq) + NO(g)
The reaction coefficient in a chemical equation shows the mole ratio of the compounds in the reactants and products
From the equation, mol ratio of NO₂ and NO = 3 : 1, so moles of NO :