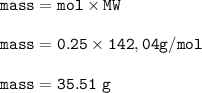The chemist weigh out 35.51 g Na2SO4
Further explanation
Given
0.250 moles of Na2SO4
Required
weight of Na2SO4
Solution
A mole is a number of particles(atoms, molecules, ions) in a substance
This refers to the atomic total of the 12 gr C-12 which is equal to 6.02.10²³, so 1 mole = 6.02.10²³ particles
Moles of a substance can also be determined from its molecular weight
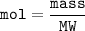
For 0.250 moles of Na2SO4, mass =