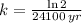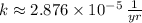Answer:
a)
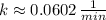 (
(
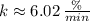 ), b)
), b)
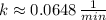 (
(
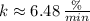 ), c)
), c)
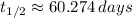 , d)
, d)
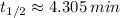 , e)
, e)
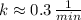 (
(
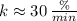 ), f)
), f)
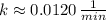 (
(
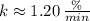 ), g)
), g)
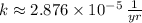 (
(
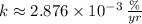 )
)
Explanation:
All radioactive isotopes decay exponentially, the mass of the isotope as a function of time (
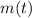 ), measured in grams, is defined by:
), measured in grams, is defined by:
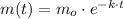 (1)
(1)
Where:
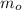 - Initial mass of the isotope, measured in grams.
- Initial mass of the isotope, measured in grams.
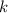 - Decay rate, measured in
- Decay rate, measured in
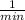 or
or
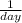 or
or
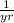 .
.
 - Time, measured in minutes, days or years.
- Time, measured in minutes, days or years.
We define the decay rate in terms of half-life by using the following expression:
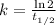 (2)
(2)
Where
 is the half-life of the isotope, measured in minutes or years.
is the half-life of the isotope, measured in minutes or years.
Now we proceed to determine the missing values:
a) Polonium-200 (
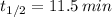 )
)
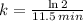
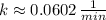
b) Lead-194 (
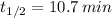 )
)
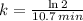
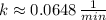
c) Iodine-125 (
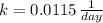 )
)
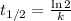
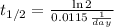
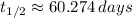
d) Kryption-75 (
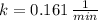 )
)
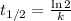
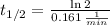
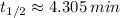
e) Strontium-79 (
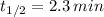 )
)
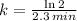
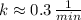
f) Uranium-229 (
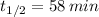 )
)
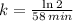
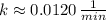
g) Plutonium-239 (
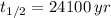 )
)