Volume of Ammonia(NH₃) = 22.4 L
Further explanation
Given
Reaction
3CuO+2NH₃⇒ 3Cu + 3H₂O+ N₂
In the problem, the CuO coefficient should be 3 not 2
M CuO = 80
mass CuO = 120 g
Required
The volume of NH₃
Solution
mol CuO :
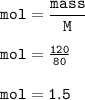
From the equation, mol ratio CuO : NH₃ = 3 : 2, so mol NH₃=
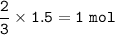
Assume at STP(0 °C, 1 atm) ⇒1 mol = 22.4 L, then volume of NH₃=22.4 L