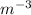Answer:
Step-by-step explanation:
The density of a substance is the ratio of its mass to its volume. Its unit of measurement is kg
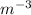 .
.
i.e density, ρ =

1a. To determine the density of the acetic acid, Rachael needs to know the mass and volume of the acid.
i. Measure the mass of the given beaker using the mass balance.
ii. Transfer the acetic acid into the beaker, and measure the new mass using the mass balance.
iii. Subtract the mass of the beaker from the new mass to determine the mass of the acetic acid.
iv. Measure the volume of the acid on the scale of the beaker.
v. Divide the value of the mass by its volume to determine its density of the acetic acid.
b. Given that the density is 1.05 g/
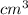 , and volume is 200
, and volume is 200
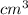 .
.
Then,
density =

1.05 =
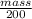
mass = 1.05 x 200
= 210 g
mass = 210 g
2. Length of titanium = 0.40 m
Area of titanium = 0.05
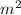
mass = 90.0 kg
density =

But,
volume = area x length
= 0.05 x 0.4
= 0.02

density of titanium =
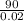
= 4500 kg