Answer:
Choice a. The compound
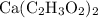 contains approximately
contains approximately
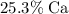 , by mass, approximately
, by mass, approximately
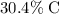 by mass, approximately
by mass, approximately
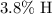 by mass, and approximately
by mass, and approximately
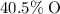 by mass.
by mass.
Step-by-step explanation:
Look up the relative atomic mass of these elements on a modern periodic table:
The relative atomic mass of an element gives the numerical value of the mass (in grams) of one mole of the atoms of this element. For example, the relative atomic mass of hydrogen is
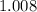 . Therefore, the mass of one mole of hydrogen atoms would be (approximately)
. Therefore, the mass of one mole of hydrogen atoms would be (approximately)
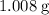 .
.
One mole of
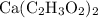 formula units include:
formula units include:
Inside each mole of
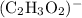 ions, there are:
ions, there are:
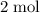 of
of
 atoms,
atoms,
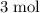 of
of
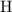 atoms, and
atoms, and
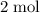 of
of
 atoms.
atoms.
Therefore, that
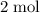 of
of
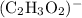 ions in one mole of
ions in one mole of
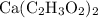 formula units would include:
formula units would include:
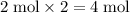 of
of
 atoms,
atoms,
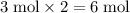 of
of
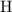 atoms, and
atoms, and
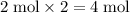 of
of
 atoms.
atoms.
In summary, each mole of
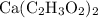 formula units would contain:
formula units would contain:
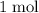 of
of
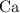 atoms,
atoms,
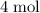 of
of
 atoms,
atoms,
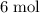 of
of
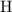 atoms, and
atoms, and
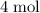 of
of
 atoms.
atoms.
Calculate the mass of these atoms using their relative atomic mass data.
For example, the mass of one mole of
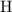 atoms is (approximately)
atoms is (approximately)
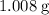 because the relative atomic mass of hydrogen is
because the relative atomic mass of hydrogen is
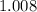 . Therefore, the mass of that
. Therefore, the mass of that
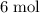 of hydrogen atoms would be approximately:
of hydrogen atoms would be approximately:
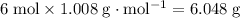 .
.
Similarly, calculate the mass of the other three elements in one mole of
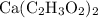 formula units:
formula units:
The sum of these masses is
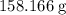 .
.
In other words, the mass of one mole of
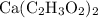 formula units is (approximately)
formula units is (approximately)
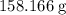 . Approximately
. Approximately
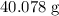 of that is
of that is
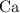 . Therefore, the percentage of
. Therefore, the percentage of
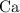 by mass in
by mass in
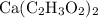 would be approximately:
would be approximately:
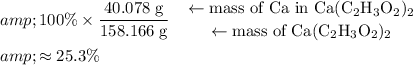 .
.
In other words,
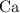 accounts for approximately
accounts for approximately
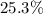 of the mass of
of the mass of
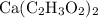 .
.
In a similar way, calculate the percentage of the other three elements by mass.
 : approximately
: approximately
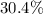 .
.
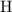 : approximately
: approximately
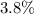 .
.
 : approximately
: approximately
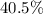 .
.