Answer:

Step-by-step explanation:
A percent yield helps measure how successful or precise a reaction was. It is ratio of measured yield to theoretical yield as a percent. The formula is:
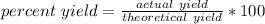
For this reaction, the measured/actual yield was 278 grams, while the theoretical yield was 365 grams.
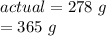
Substitute the values into the formula.
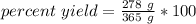
Divide first. The grams (g) will cancel out.
- 278 g/365 g= 278/365=0.7616438356
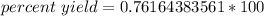
Multiply.
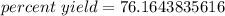
The original measurements both had 3 significant figures, so we should round our percent yield to 3 sig figs. In this case, that is the tenths place.
The 6 in the tenth place tells us to round the 1 up to a 2.
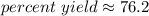
The percent yield is about 76.2 %