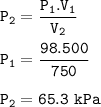500 mL of He at 98 kPa expands to 750 mL. Find P2.
P₂ = 65.3 kPa
Further explanation
Given
V₁=500 ml
P₁=98 kPa
V₂=750 ml
Required
P₂
Solution
Boyle's Law
At a constant temperature, the gas volume is inversely proportional to the pressure applied
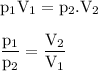
Input the value :