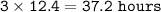The number of hours required : 37.2 hours
Further explanation
Given
⁴²K (potassium -42)
Required
The number of hours
Solution
The atomic nucleus can experience decay into 2 particles or more due to the instability of its atomic nucleus.
Usually, radioactive elements have an unstable atomic nucleus.
Based on Table N(attached), the half-life for ⁴²K is 12.4 hours, which means half of a sample of ⁴²K will decay in 12.4 hours
For three half-life periods :