Answer:
S = 2461.53 [kJ]
Step-by-step explanation:
The change in entropy in a process such as melting can be calculated by means of the following expression.
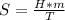
where:
S = entropy [kJ]
H = fusion heat = 3.36*10¹ [J/kg]
m = mass = 0.02 [kg]
T = temperature in kelvin = 273 [K]
![S = (0.02*3.36*10^(1) )/((273+0))\\S = 2461.53 [kJ]](https://img.qammunity.org/2021/formulas/physics/college/abkot0usf2unnvvdvpqf97ombynfwzzgww.png)