If the atom has no charge (neutral) then there are the same number of protons and electrons. The positive protons cancel out with the negative electrons to produce this neutral state. We can say the net charge is 0 here.
Now if you take away one or more electrons, then there will be more positive protons to outnumber the negative electrons. Overall, the net charge will be positive. For example, if the atom loses 3 electrons, then you'll have a net charge of
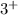 . These positively charged ions are known as cations. You pronounce this as "cat-ion" instead of "catshun".
. These positively charged ions are known as cations. You pronounce this as "cat-ion" instead of "catshun".
On the flip side, if you add electrons to an atom, then you'll make the net charge overall to be negative. So let's say we add 4 electrons. We'll have the net charge be
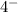 and this is an anion. To pronounce "anion" you would say "an ion" instead of "anyun".
and this is an anion. To pronounce "anion" you would say "an ion" instead of "anyun".
- cation = positively charged ion (less electrons compared to protons)
- anion = negatively charged ion (more electrons compared to protons)
---------------
In short, the number of protons and electrons are the same if the net charge is 0 and we're not dealing with ions. If you are dealing with ions, then the two counts will not be the same. Note how in the examples above, I didn't change the number of protons. Doing so would change the overall element entirely.