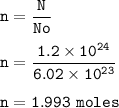The number of moles of Hydrogen : 1.993
Further explanation
Given
1.20 x 10²⁴ hydrogen atoms
Required
The number of moles
Solution
The mole is the number of particles(molecules, atoms, ions) contained in a substance
1 mol = 6.02.10²³ particles
Can be formulated :
N=n x No
N = number of particles
n = mol
No = Avogadro's = 6.02.10²³
moles of Hydrogen :