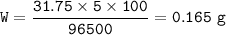Mass of copper : 0.165 g
Further explanation
Given
5.0 A over 100 seconds
Required
Mass of copper
Solution
Faraday's law:
The mass of the substance formed at each electrode is proportional to the electric current flowing in the electrolysis
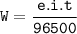
e = Ar / valence = eqivalent weight
i = current
t = time
W = weight
CuSO₄ ----> Cu²⁺ + SO₄²⁻
Cu ----> Cu²⁺ + 2e
e = Ar/2
= 63,5/2 = 31,75