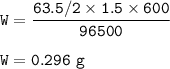The mass of Copper deposited at the cathode : 0.296 g
Further explanation
Given
time = t = 10 min=600 s
current = i = 1.5 A
F = 96500 C
charge Cu=+2
Required
The mass of Copper
Solution
Faraday's Law
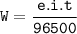
e = Ar/valence(valence Cu=2, Ar=63.5 g/mol)
Input the value :