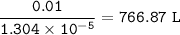The volume of water = 766.87 L
Further explanation
Given
The K for AgCl is 1.7x10⁻¹⁰
Required
the volume of water
Solution
AgCl dissolves in water to form ions(s=solubility)
AgCl ⇒ Ag⁺ + Cl⁻
s s s
K AgCl = [Ag⁺] [Cl⁻]
K AgCl = (s) (s) = s²
1.7x10⁻¹⁰ = s²
s=1.304 x 10⁻⁵ mol/L
The solubility of AgCl in water = 1.304 x 10⁻⁵ mol/L
So for 0.01 moles of AgCl, water needed :