Answer:
a. 3.72 [atm]
Step-by-step explanation:
For a gas at constant temperature, (with no change in number of molecules of the gas), we can apply Boyle's Law:
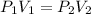
![(1.556[atm])(268.5[mL])=P_2(112.4[mL])](https://img.qammunity.org/2023/formulas/chemistry/high-school/796e4mm9k3g1lurcn95jrr1nb9aiw36811.png)
![\frac{(1.556[atm])(268.5[mL\!\!\!\!\!\!\!\!{--}])}{112.4[mL \!\!\!\!\!\!\!\!{--}]}=\frac{P_2(112.4[mL]\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!{-----})}{112.4[mL]\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!{-----}}](https://img.qammunity.org/2023/formulas/chemistry/high-school/nmolvjdm81kjaw2nuamb5sxyvrkyzhk00q.png)
![3.716957[atm]=P_2](https://img.qammunity.org/2023/formulas/chemistry/high-school/563i26mxyq303k7pcumtczgqqjmjf7sdf5.png)
It seems like the answer should have 4 significant figures since all of the other quantities have 4 significant figures, but the closest answer choice of those provided is a. 3.72