Answer:
a.
![P_2=1.38*10^4[mmHg]](https://img.qammunity.org/2023/formulas/chemistry/high-school/kx0fn6b7lgyspjpsz2o66k78tlg3a8nv66.png)
Step-by-step explanation:
If the Temperature of a gas remains constant (and if the amount of gas molecules doesn't change), then compressing the gas from 12.11 L to 0.669L will increase its pressure through Boyle's Law:
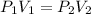
We'll also need to recall that atmospheric pressure with units of mmHg (since that is the unit requested in the answer) is 760 mmHg.
Letting the initial Pressure and Volume be the P1 and V1, and the final pressure be the P2 and V2, we can substitute and solve:
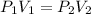
![(760[mmHg])(12.11[L])=P_2(0.669[L])](https://img.qammunity.org/2023/formulas/chemistry/high-school/hdn4qiuhziy774nfxz7w78xw01xptyqoyu.png)
![((760[mmHg])(12.11[L \!\!\!\!-]))/(0.669[L \!\!\!\!-])=\frac{P_2(0.669[L]\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!{----})}{0.669[L]\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!{----}}](https://img.qammunity.org/2023/formulas/chemistry/high-school/o63uecw9sttvcxn4dyv411y0umh17c82re.png)
![13757.2496[mmHg]=P_2](https://img.qammunity.org/2023/formulas/chemistry/high-school/y3o4f0ok2dcobyl2t8057eonwuaw93083b.png)
Since the final pressure is only measured to 3 significant figures, we round the pressure accordingly
![13800[mmHg]=P_2](https://img.qammunity.org/2023/formulas/chemistry/high-school/4jbqzxu5bhxjnkvdyyb8z17kcweauj2obt.png)
Note that in scientific notation, this is
![P_2=1.38*10^4[mmHg]](https://img.qammunity.org/2023/formulas/chemistry/high-school/kx0fn6b7lgyspjpsz2o66k78tlg3a8nv66.png) , occasionally written as
, occasionally written as
![1.38\text{E}4[mmHg]](https://img.qammunity.org/2023/formulas/chemistry/high-school/ruvavxq59t2j6m7jejfhzl24ztrgijvb8y.png)