Answer:
a. P=188mmHg
Step-by-step explanation:
A gas undergoing a change of volume and pressure at constant temperature (and not changing the number of molecules), follows Boyle's Law:
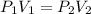
The units of these quantities can be any measure of pressure and volume, so long as long as the units are the same between beginning and ending conditions.
Substitute and solve:
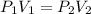
![(685[mmHg])(131[mL])=P_2(478[mL])](https://img.qammunity.org/qa-images/2023/formulas/chemistry/high-school/wg5o1qqxelfo0mdre26y.png)
![\frac{(685[mmHg])(131[mL\!\!\!\!\!\!\!\!\!{--}])}{478[mL\!\!\!\!\!\!\!\!\!{--}]}=\frac{P_2(478[mL]\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!{----})}{478[mL] \!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!{----}}](https://img.qammunity.org/qa-images/2023/formulas/chemistry/high-school/txgi4ypwhoera3p2vqwu.png)
![187.730125523[mmHg]=P_2](https://img.qammunity.org/qa-images/2023/formulas/chemistry/high-school/1dv81xabdjmm1yvfnrm4.png)
Accounting for significant figures,
![P_2=188[mmHg]](https://img.qammunity.org/qa-images/2023/formulas/chemistry/high-school/7t2ix5uegu6jrsuiz68x.png)