Answer:
a. P = 320 mmHg
Step-by-step explanation:
For this problem, the pressure, volume, and temperature are changing, so we'll need to combine Boyle's Law and Charles' Law:
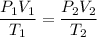
For this equation, the temperatures must be measured in Kelvin. The rest of units in the equation only need to match between beginning and end conditions.
Recall that to convert from Celsius to Kelvin, add 273, or use the equation
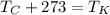 .
.
So
![T_1=(44.4+273)[K]=317.4[K]](https://img.qammunity.org/2023/formulas/chemistry/high-school/pt2w684pjia8h8o2va63dlw7mq2shct1pp.png) and
and
![T_2=(68.1+273)[K]=341.1[K]](https://img.qammunity.org/2023/formulas/chemistry/high-school/lbgysca0ye6ys0478uuv6oev0y8ob411xt.png)
Substituting known values, we can solve for the unknown:
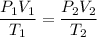
![((574.6[mmHg])(208.1[mL]))/((317.4[K]))=(P_2(401.5[mL]))/((341.1[K]))](https://img.qammunity.org/2023/formulas/chemistry/high-school/kfdh0kged6mek71hpqrw3yr5omzu6rvaq7.png)
![\frac{(574.6[mmHg])(208.1[mL]\!\!\!\!\!\!\!\!\!\!{--} )}{317.4[K]\!\!\!\!\!\!{-}}*\frac{341.1[K] \!\!\!\!\!\!{-}}{401.5[mL] \!\!\!\!\!\!\!\!\!\!\!{--} }=\frac{P_2(401.5[mL] \!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!{-----})}{341.1[K] \!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!{----}}*\frac{341.1[K] \!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!{----}}{401.5[mL] \!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!{-----} }](https://img.qammunity.org/2023/formulas/chemistry/high-school/6wku4vax5nbmvekw5ek3oczxqh8mxve066.png)
![320.056719296[mmHg]=P_2](https://img.qammunity.org/2023/formulas/chemistry/high-school/ak2hcrj2e8ssttt9la153nxoacpq294i37.png)
Accounting for significant figures,
![P_2=320.1[mmHg]](https://img.qammunity.org/2023/formulas/chemistry/high-school/7bxwqm2e3c58b2b3ss0uvlz79k3gnh6l6e.png) .
.
The closest answer provided is 320, so "a".