Answer:
a. 1810mL
Step-by-step explanation:
When conditions for a gas change under constant pressure (and the number of molecules doesn't change), it follows Charles' Law:
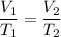 where the temperatures must be measured in Kelvin
where the temperatures must be measured in Kelvin
To convert from Celsius to Kelvin, add 273, or use the equation:
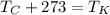
For this problem, one must also recall that standard temperature is 0°C (or 273K).
So,
![T_1 = 273[K]](https://img.qammunity.org/2023/formulas/chemistry/high-school/5vjopby3oct94cthlby48q9fnthftkry4r.png) , and
, and
![T_2 = (49.4+273)[K]=322.4[K]](https://img.qammunity.org/2023/formulas/chemistry/high-school/3xg33b9qe1xtc0ddnrxolnw64da8tstw09.png) .
.
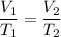
![((1532.7[mL]))/((273[K]))=(V_2)/((322.4[K]))](https://img.qammunity.org/2023/formulas/chemistry/high-school/5pshhf9we7ezdj1sow640chxkp39s4ho27.png)
![\frac{(1532.7[mL])}{(273[K\!\!\!\!\!{-}])}(322.4[K\!\!\!\!\!{-}] )=\frac{V_2}{(322.4[K]\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!{----})}(322.4[K]\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!{----})](https://img.qammunity.org/2023/formulas/chemistry/high-school/6fplv5owblc08vqiuzsvh1z42lssa2cblq.png)
![1810.04571428[mL]=V_2](https://img.qammunity.org/2023/formulas/chemistry/high-school/px02exgh4zvkn53i4mj3o6tlrrzuxpsj5w.png)
Adjusting for significant figures, this gives
![V_2=1810[mL]](https://img.qammunity.org/2023/formulas/chemistry/high-school/volq1s2j4u1xsovnps5ofy26xgkpc5bmxy.png)