Limiting reactant : HNO₃
Further explanation
Given
150 ml 25 g NaOH
150 ml 25 g HNO₃
Required
the limiting reactant
Solution
Reaction
NaOH+HNO₃⇒NaNO₃ + H₂O
Find mol
mol NaOH (MW=40 g/mol):
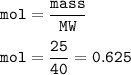
mol HNO₃(MW=63 g/mol) :
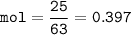
A method that can be used to find limiting reactants is to divide the number of moles of known substances by their respective coefficients, and small or exhausted reactants become limiting reactants
Because from the equation, mol ratio NaOH : HNO₃, so HNO₃ becomes a limiting reactant