Answer:
The new pressure becomes one third of the initial pressure.
Step-by-step explanation:
The relation between pressure and volume at constant temperature is given by :
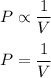
Let new pressure and volume be P' and V' respectively.
V'=3V (given)
So,
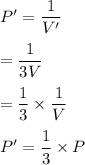
Hence, new pressure becomes one third of the initial pressure.