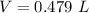Complete Question
The complete question is shown on the first uploaded image
Answer:
Step-by-step explanation:
From the question we are told that
The initial pressure of the gas is
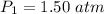
The volume of the second bulb is
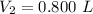
The final pressure of the gas is
Let the unknown volume be represented as
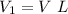
Generally from Boyle's law we have that
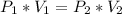
=>
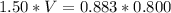
=>