Answer:
The spectator ions is:
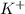 and
and
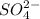
Step-by-step explanation:
The equation of reaction between H₂ SO₄ and KOH is:
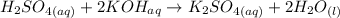
Rewriting this equation as ionic;
![[2H^(+) + SO^(2-)_4 + 2K^+ +2OH^- \to 2K^+ SO_4^(2-) + 2H_2O ]](https://img.qammunity.org/2021/formulas/chemistry/college/c252e8r9ey6jfa84x8sx475vt7aentprra.png)
Spectators ions are ions present on both sides of the ionic equation by the same quantity but do not take part in the net reaction.