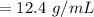Answer:
"12.4 g/mL" would be the right solution.
Step-by-step explanation:
The given values are:
Mass of the object,
= 149.8 g
Volume of the object,
= 12.1 mL
Now,
The Density will be:
⇒
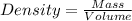
On substituting the given values in the above formula, we get
⇒
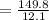
⇒