Answer:
mass of CO2 = 20.9 g
Step-by-step explanation:
Given that:
mass of O2= 30.4 g
molar mass O2 = 32
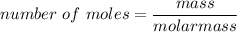
number of moles of O2 = 30.4 / 32
number of moles of O2 = 0.95 moles
The Volume of O2 = 2V
Using ideal gas equation:
PV = nRT
P × (2V) = 0.95 × R × T
PV = 0.475 RT ----- (1)
For CO2;
The volume = V
Using ideal gas equation:
PV = nRT ---- (2)
Equating equation (1) and (2) together, we get:
0.475 RT = nRT
Dividing both sides by RT
(0.475 RT)/RT = (nRT)/RT
0.475 = n
Thus, the number of moles of CO2 = 0.475
Since mass = number of moles × molar mass
mass of CO2 = 0.475 × 44
mass of CO2 = 20.9 g